Occupational
Exposure to asbestos fibers has been recognized as an occupational health hazard since the early 20th century. Numerous epidemiological studies have associated occupational exposure to asbestos with the development of pleural plaques, diffuse pleural thickening, asbestosis, carcinoma of the lung and larynx, gastrointestinal tumors, and diffuse malignant mesothelioma of the pleura and peritoneum. Asbestos has been widely used in many industrial products, including cement, brake linings, gaskets, roof shingles, flooring products, textiles, and insulation.Commercial asbestos mining at Wittenoom, Western Australia, occurred between 1945 and 1966. A cohort study of miners employed at the mine reported that while no deaths occurred within the first 10 years after crocidolite exposure, 85 deaths attributable to mesothelioma had occurred by 1985. By 1994, 539 reported deaths due to mesothelioma had been reported in Western Australia.
Paraoccupational secondary exposure
Family members and others living with asbestos workers have an increased risk of developing mesothelioma, and possibly other asbestos related diseases.[24][25] This risk may be the result of exposure to asbestos dust brought home on the clothing and hair of asbestos workers. To reduce the chance of exposing family members to asbestos fibres, asbestos workers are usually required to shower and change their clothing before leaving the workplace.Asbestos in buildings
Many building materials used in both public and domestic premises prior to the banning of asbestos may contain asbestos. Those performing renovation works or DIY activities may expose themselves to asbestos dust. In the UK use of Chrysotile asbestos was banned at the end of 1999. Brown and blue asbestos was banned in the UK around 1985. Buildings built or renovated prior to these dates may contain asbestos materials.Diagnosis
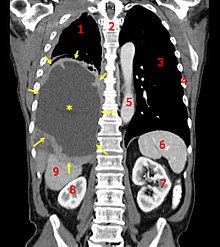
CT scan of a patient with mesothelioma, coronal section
(the section follows the plane that divides the body in a front and a
back half). The mesothelioma is indicated by yellow arrows, the central pleural effusion (fluid collection) is marked with a yellow star. Red numbers: (1) right lung, (2) spine, (3) left lung, (4) ribs, (5) descending part of the aorta, (6) spleen, (7) left kidney, (8) right kidney, (9) liver.

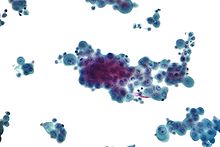
Micrographs showing mesothelioma in a core biopsy.
Generally, a biopsy is needed to confirm a diagnosis of malignant mesothelioma. A doctor removes a sample of tissue for examination under a microscope by a pathologist. A biopsy may be done in different ways, depending on where the abnormal area is located. If the cancer is in the chest, the doctor may perform a thoracoscopy. In this procedure, the doctor makes a small cut through the chest wall and puts a thin, lighted tube called a thoracoscope into the chest between two ribs. Thoracoscopy allows the doctor to look inside the chest and obtain tissue samples. Alternatively, the chest surgeon might directly open the chest (thoracotomy). If the cancer is in the abdomen, the doctor may perform a laparoscopy. To obtain tissue for examination, the doctor makes a small incision in the abdomen and inserts a special instrument into the abdominal cavity. If these procedures do not yield enough tissue, more extensive diagnostic surgery may be necessary.
Immunohistochemical studies play an important role for the pathologist in differentiating malignant mesothelioma from neoplastic mimics. There are numerous tests and panels available. No single test is perfect for distinguishing mesothelioma from carcinoma or even benign versus malignant. Currently, there is no FDA approved immunohistochemistry assay for the diagnosis of mesothelioma so different cancer centers use different panels.
| Positive | Negative |
| EMA (epithelial membrane antigen) in a membranous distribution | CEA (carcinoembryonic antigen) |
| WT1 (Wilms' tumour 1) | B72.3 |
| Calretinin[5] | MOC-3 1 |
| Mesothelin-1 | CD15 |
| Cytokeratin 5/6[5] | Ber-EP4 |
| HBME-1 (human mesothelial cell 1) | TTF-1 (thyroid transcription factor-1) |
Staging
Staging of mesothelioma is based on the recommendation by the International Mesothelioma Interest Group.[27] TNM classification of the primary tumor, lymph node involvement, and distant metastasis is performed. Mesothelioma is staged Ia–IV (one-A to four) based on the TNM status.[27][28]Screening
There is no universally agreed protocol for screening people who have been exposed to asbestos. Screening tests might diagnose mesothelioma earlier than conventional methods thus improving the survival prospects for patients. The serum osteopontin level might be useful in screening asbestos-exposed people for mesothelioma. The level of soluble mesothelin-related protein is elevated in the serum of about 75% of patients at diagnosis and it has been suggested that it may be useful for screening.[29] Doctors have begun testing the Mesomark assay which measures levels of soluble mesothelin-related proteins (SMRPs) released by diseased mesothelioma cells.[30]Pathophysiology
The mesothelium consists of a single layer of flattened to cuboidal cells forming the epithelial lining of the serous cavities of the body including the peritoneal, pericardial and pleural cavities. Deposition of asbestos fibers in the parenchyma of the lung may result in the penetration of the visceral pleura from where the fiber can then be carried to the pleural surface, thus leading to the development of malignant mesothelial plaques. The processes leading to the development of peritoneal mesothelioma remain unresolved, although it has been proposed that asbestos fibers from the lung are transported to the abdomen and associated organs via the lymphatic system. Additionally, asbestos fibers may be deposited in the gut after ingestion of sputum contaminated with asbestos fibers.Pleural contamination with asbestos or other mineral fibers has been shown to cause cancer. Long thin asbestos fibers (blue asbestos, amphibole fibers) are more potent carcinogens than "feathery fibers" (chrysotile or white asbestos fibers).[8] However, there is now evidence that smaller particles may be more dangerous than the larger fibers. They remain suspended in the air where they can be inhaled, and may penetrate more easily and deeper into the lungs. "We probably will find out a lot more about the health aspects of asbestos from [the World Trade Center attack], unfortunately," said Dr. Alan Fein, chief of pulmonary and critical-care medicine at North Shore-Long Island Jewish Health System. Dr. Fein has treated several patients for "World Trade Center syndrome" or respiratory ailments from brief exposures of only a day or two near the collapsed buildings.[31]
Mesothelioma development in rats has been demonstrated following intra-pleural inoculation of phosphorylated chrysotile fibers. It has been suggested that in humans, transport of fibers to the pleura is critical to the pathogenesis of mesothelioma. This is supported by the observed recruitment of significant numbers of macrophages and other cells of the immune system to localized lesions of accumulated asbestos fibers in the pleural and peritoneal cavities of rats. These lesions continued to attract and accumulate macrophages as the disease progressed, and cellular changes within the lesion culminated in a morphologically malignant tumor.
Experimental evidence suggests that asbestos acts as a complete carcinogen with the development of mesothelioma occurring in sequential stages of initiation and promotion. The molecular mechanisms underlying the malignant transformation of normal mesothelial cells by asbestos fibers remain unclear despite the demonstration of its oncogenic capabilities (see next-but-one paragraph). However, complete in vitro transformation of normal human mesothelial cells to malignant phenotype following exposure to asbestos fibers has not yet been achieved. In general, asbestos fibers are thought to act through direct physical interactions with the cells of the mesothelium in conjunction with indirect effects following interaction with inflammatory cells such as macrophages.
Analysis of the interactions between asbestos fibers and DNA has shown that phagocytosed fibers are able to make contact with chromosomes, often adhering to the chromatin fibers or becoming entangled within the chromosome. This contact between the asbestos fiber and the chromosomes or structural proteins of the spindle apparatus can induce complex abnormalities. The most common abnormality is monosomy of chromosome 22. Other frequent abnormalities include structural rearrangement of 1p, 3p, 9p and 6q chromosome arms.
Common gene abnormalities in mesothelioma cell lines include deletion of the tumor suppressor genes:
- Neurofibromatosis type 2 at 22q12
- P16INK4A
- P14ARF
- Inactivation of tumor suppressor genes
- Activation of oncogenes
- Activation of proto-oncogenes due to incorporation of foreign DNA containing a promoter region
- Activation of DNA repair enzymes, which may be prone to error
- Activation of telomerase
- Prevention of apoptosis
Asbestos also may possess immunosuppressive properties. For example, chrysotile fibres have been shown to depress the in vitro proliferation of phytohemagglutinin-stimulated peripheral blood lymphocytes, suppress natural killer cell lysis and significantly reduce lymphokine-activated killer cell viability and recovery. Furthermore, genetic alterations in asbestos-activated macrophages may result in the release of potent mesothelial cell mitogens such as platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β) which in turn, may induce the chronic stimulation and proliferation of mesothelial cells after injury by asbestos fibres.

Aucun commentaire:
Enregistrer un commentaire